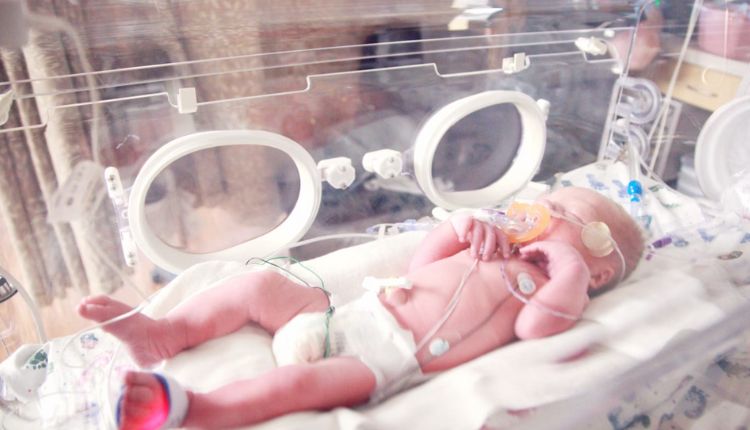
In the world of infant nutrition, Enfamil, a trusted brand for decades, has found itself entangled in a web of lawsuits. These cases are centered around a severe gastrointestinal illness—necrotizing enterocolitis (NEC). This alarming trend has led parents and caregivers to question the safety of Enfamil baby formula.
As legal battles unfold, the fallout resonates across neonatal intensive care units (NICUs) and homes. This has raised concerns and prompted a closer examination of the connection between Enfamil and NEC.
In this article, we will dive into the complexities surrounding the controversy. We will shed light on ongoing legal proceedings, the science behind the allegations, and the dedicated advocacy emerging on behalf of premature infants.
The Enfamil Lawsuits
The genesis of the Enfamil lawsuit lies in the heart-wrenching experiences of parents of infants who have suffered terribly. These parents claim that Mead Johnson’s Enfamil baby formula failed to provide adequate warnings about the potential risks associated with necrotizing enterocolitis (NEC).
According to TorHoerman Law, allegations suggest that the manufacturer was aware of a heightened risk of NEC in preterm infants. However, they failed to disclose this critical information to consumers. As a result, parents were blindsided by the severe gastrointestinal illness affecting their vulnerable infants.
Legal proceedings began as a response to mounting cases of NEC in infants who had been fed Enfamil. Questions about product safety and the responsibility of the manufacturer have been raised since then.
The lawsuits underscore the crucial need for transparency in communicating potential health risks to consumers, particularly when it concerns the well-being of newborns.
The Link Between Enfamil and NEC
Scientific research dives into studies dating back to 1990, revealing a concerning pattern. Drugwatch notes that infants exclusively fed formula, such as Enfamil, were found to be six to ten times more likely to develop NEC. This is in comparison to their breastfed counterparts.
The frequency of NEC in formula-fed preemies, coupled with the rarity of the condition in breastfed infants, forms a compelling foundation for the lawsuits. Critics argue that Mead Johnson was cognizant of these scientific findings yet continued to market Enfamil without adequately warning consumers about the potential risks.
Understanding the scientific underpinnings is integral to assessing the validity of the claims. It is also critical to determine the extent to which Enfamil may have played a role in the development of NEC in preterm infants.
Personal Narratives Amidst Legal Battles
Amidst the legal battles, poignant narratives emerge from families who have experienced the devastating aftermath of NEC in their premature infants after Enfamil consumption. These personal stories highlight the profound emotional and financial hardships faced by parents and caregivers.
According to AboutLawsuits, one such case involves Sasha Stewart, whose premature infant developed NEC shortly after transitioning to Enfamil in the NICU. The infant’s subsequent struggles with complications, hospitalizations, and multiple surgeries underscore the gravity of the situation.
These personal accounts provide a human dimension to the legal proceedings, emphasizing the urgency of addressing potential risks associated with Enfamil.
Tracking Enfamil Lawsuits in 2024
In the legal arena, Enfamil lawsuits have coalesced into multidistrict litigation in the Northern District of Illinois. The MDL is being overseen by the U.S. District Judge Rebecca Pallmeyer.
ConsumerNotice notes that, as of March 2024, 405 lawsuits related to both Enfamil and Similac are pending in this multidistrict litigation. Despite the substantial number of cases, the litigation is still in its early stages, with no settlements or bellwether trials reported.
Negotiations regarding the discovery process are ongoing. The selection of potential bellwether test trial cases and the randomness in the selection process hint at the complexity of the litigation. Estimates suggest that trials may potentially take place this year.
A Comprehensive Product Analysis
The scope of Enfamil lawsuits extends across various product lines, encompassing a wide array of baby formulas and supplements. Among the formulas named in the lawsuits are Enfamil 24 Calorie Formula, Enfamil NeuroPro EnfaCare Premature Baby Formula, Enfamil Enspire, and numerous others.
This comprehensive product analysis reveals the diversity of Enfamil’s formula offerings implicated in the lawsuits. This raises questions about the potential risks associated with the entire product line.
Advocating for Change and Consumer Awareness
As Enfamil lawsuits progress, an emergent theme revolves around advocating for change and raising consumer awareness. The legal developments within the Enfamil lawsuits have the potential to set precedents and shape industry practices. This will influence how manufacturers communicate risks associated with their products.
Consumer advocacy groups and legal professionals are actively working to ensure that parents and caregivers are informed about potential risks when choosing infant formulas. This heightened awareness aims to empower consumers to make well-informed decisions about the nutritional choices for their infants.
The evolving landscape underscores the importance of transparency, accountability, and proactive measures to safeguard the well-being of vulnerable preterm infants. As legal proceedings unfold, the impact on formula manufacturers remains a focal point in the broader advocacy for the welfare of premature infants.
In essence, the Enfamil lawsuits underscore the imperative for transparency and accountability in the infant formula industry. As legal proceedings progress, the intersection of personal narratives and scientific findings intensifies the urgency for change.
The multidistrict litigation, with its substantial number of pending cases, becomes a focal point for reshaping industry practices. Beyond legal ramifications, ongoing advocacy efforts signal a collective determination to empower parents and caregivers with vital information.
The Enfamil controversy prompts a wider reflection on the welfare of premature infants. This emphasizes the need for proactive measures and heightened awareness in the ongoing pursuit of safeguarding the vulnerable and ensuring responsible product communication.